TTFields therapy shows great potential for versatile clinical application across solid tumors
Dive deeper into the enhanced effects of Tumor Treating Fields when used with other cancer modalities.
TTFields are electric fields that exert physical forces to kill cancer cells via a variety of mechanisms1-7:
- Electric fields have different effects on the human body depending on their frequency, leading to diverse applications in healthcare such as microwave ablation, deep brain stimulation, and pacemakers1,8-10
- TTFields employ electric fields at a frequency range of 100 kHz to 500 kHz. Its relatively high frequency range and low intensity allow it to avoid depolarizing nerves or muscle or have significant heating effects1,11
- The unique frequency range of TTFields allows the electric fields to be generated through the cancer cell membrane, while a lower frequency would not1,11
- Once the electric fields have penetrated the cancer cell they may affect polar cellular components within, potentially leading to cancer cell death1-5
- TTFields spare healthy cells because these cells have different electrical properties relative to cancer cells, differences that become more pronounced at higher stages of malignancy1,4,5,10,12,13
The multiple, distinct mechanisms of TTFields therapy work together to selectively target and kill cancer cells1-5
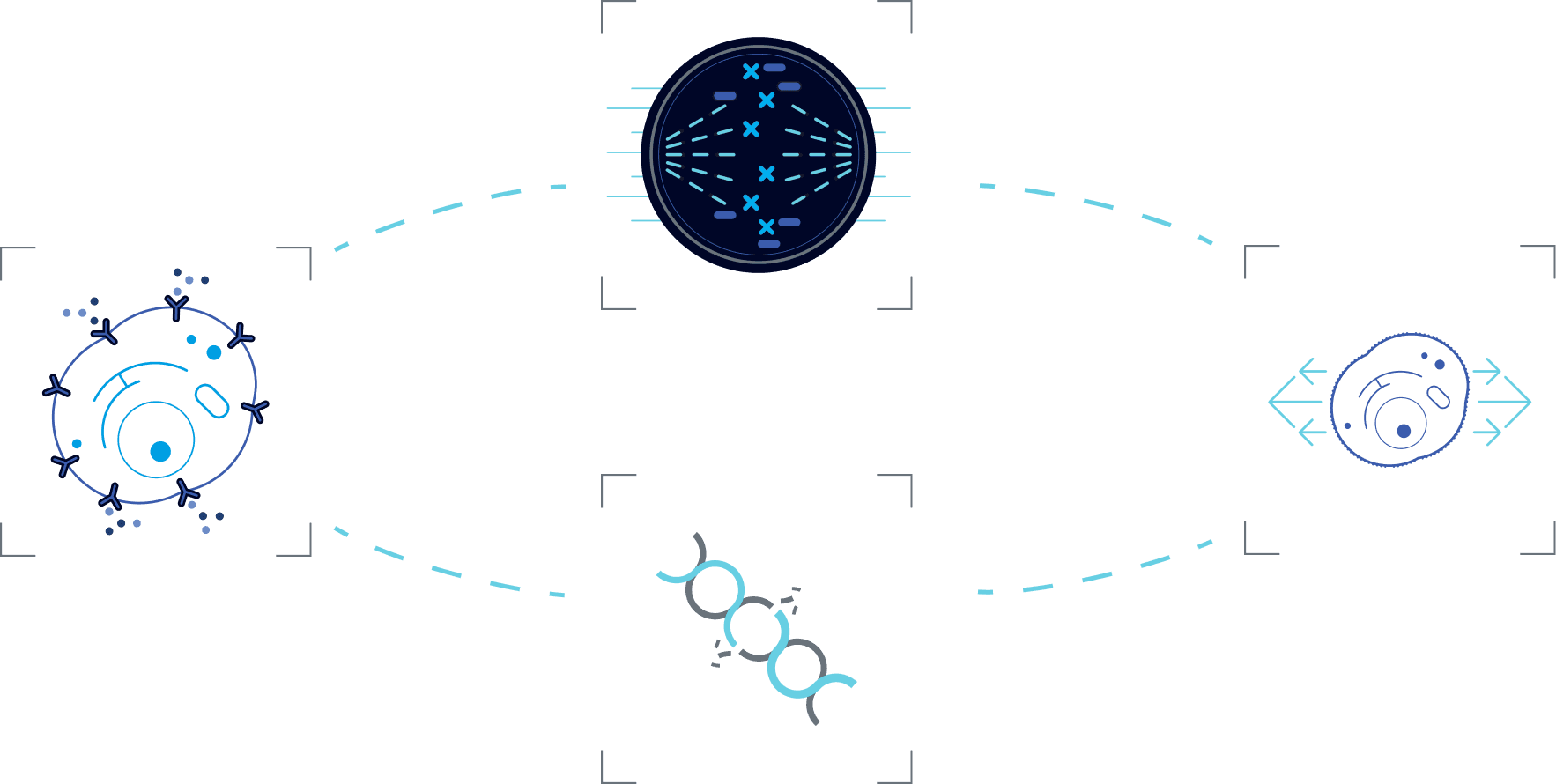
Select a mechanism to learn more about its function
In preclinical models, TTFields have been shown to disrupt mitosis by exerting electrical forces on polar components involved in this process (e.g. spindle microtubules), thus disrupting their normal localization and function, ultimately leading to cell death.1-3
Explore each phase of mitosis to see the impact of TTFields
In metaphase:
TTFields impair microtubule assembly in cancer cells, leading to aberrant mitotic spindle formation.2,4
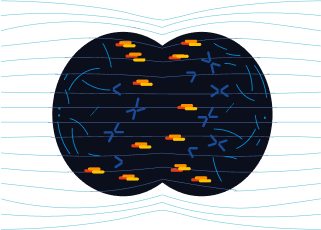
In anaphase:
TTFields disrupt the arrangement of septin at the anaphase cleavage furrow in cancer cells, inducing cytoplasmic membrane blebbing, mitotic failure, and asymmetric chromosome segregation.1,5
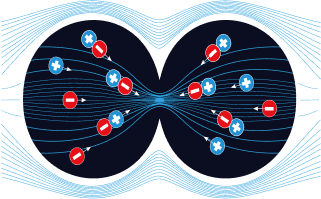
In telophase:
TTFields push polar organelles and macromolecules (a process called dielectrophoresis) to the bridge between the forming daughter cells, where - due to the hourglass shape - TTFields intensity is highest4,5
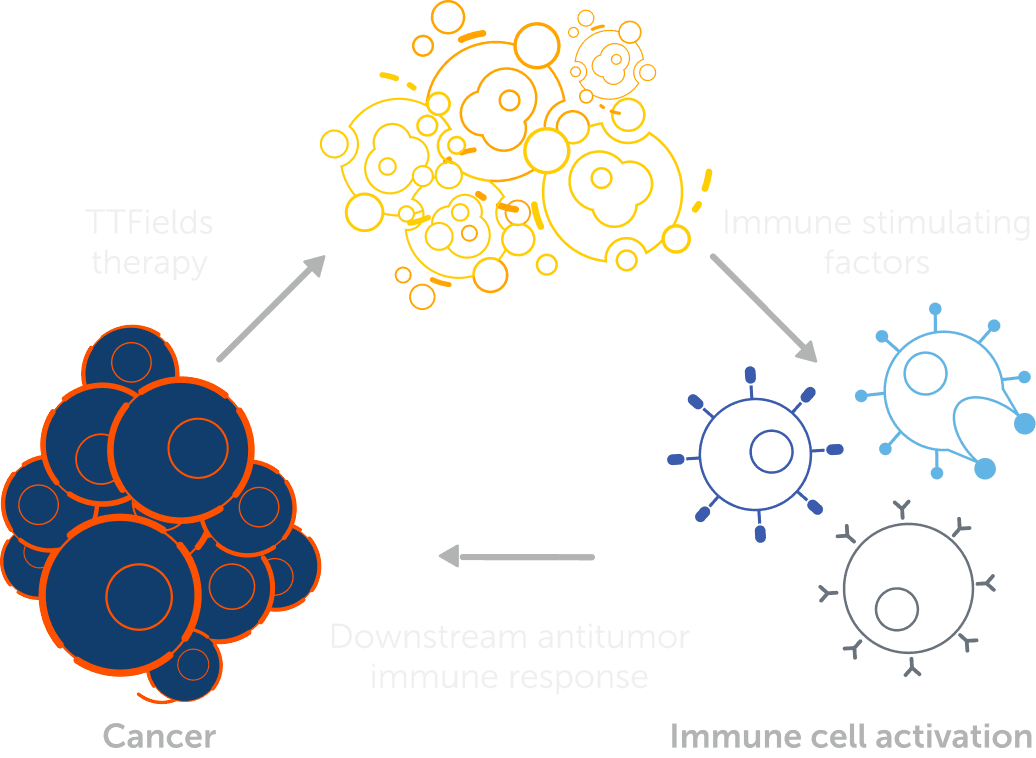

In preclinical models, TTFields have been shown to induce immunogenic cell death of cancer cells (e.g. release of HMGB1 from the nucleus, cell surface exposure of calreticulin, and autophagy-mediated ATP release), thus triggering an anti-cancer immune response
In preclinical models, TTFields have been shown to alter the organization and dynamics of the cytoskeleton, disrupting cancer cell mobility and migration, which are essential for metastasis.1
A model illustrating the mechanism by which TTFields modulates cancer cell mobility
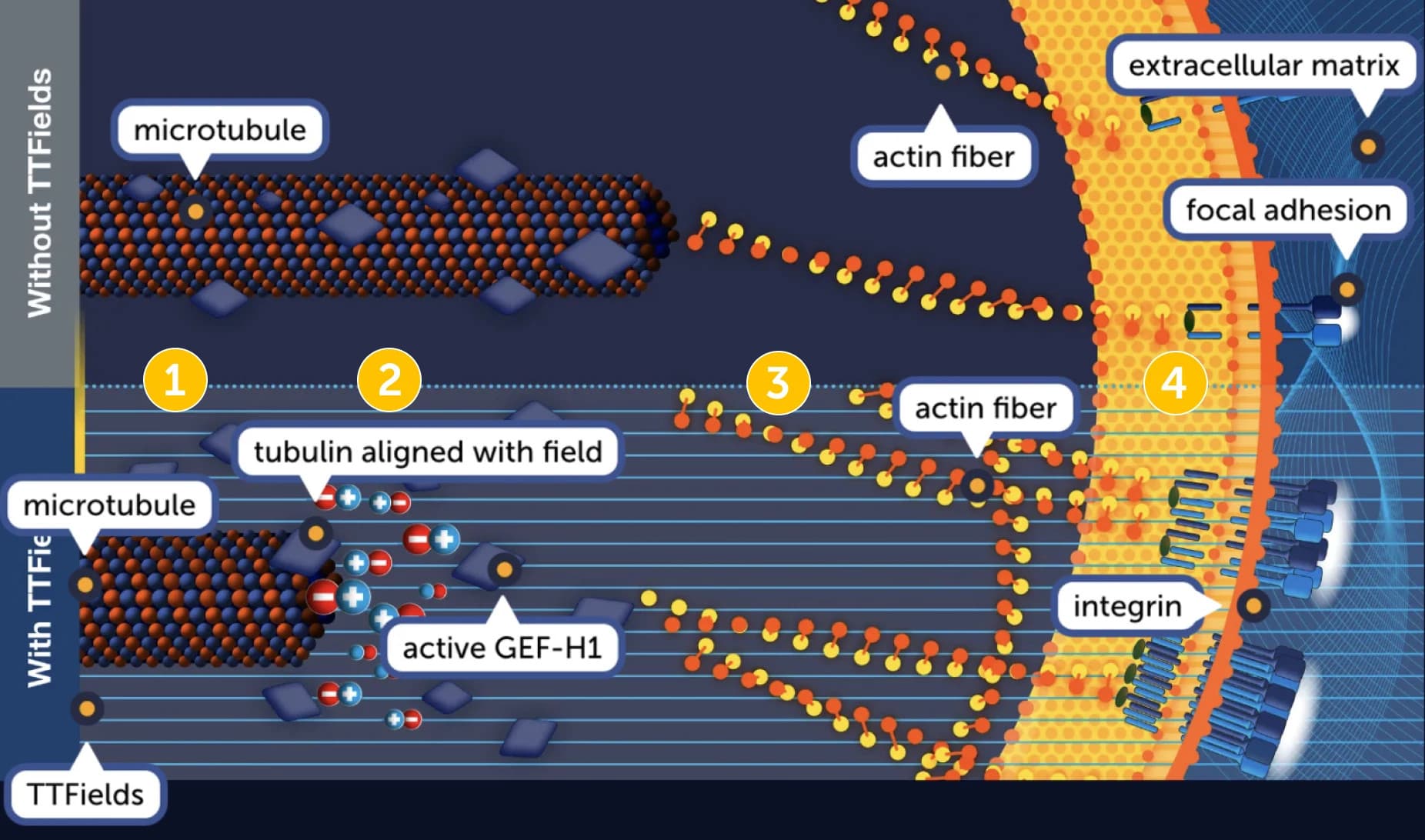
- Microtubules are required to specify the direction of cell movement. GEF-H1 catalytic activity is downregulated through microtubule binding.1
- TTFields exert directional forces on polar tubulins, leading to their alignment in the direction of the field. This in turn, leads to the reorganization of the microtubule network, and release of GEF-H1.
- Free GEF-H1 activates the RhoA/ROCK signaling pathway, inducing changes in actin dynamics.
- Changes in actin dynamics induce formation of focal adhesions, which disrupt polarity and migration directionality.
In preclinical models, TTFields have been shown to downregulate expression of proteins from the FA-BRCA pathway, an important DNA damage response pathway
Watch below to see how TTFields disrupt DNA damage repair
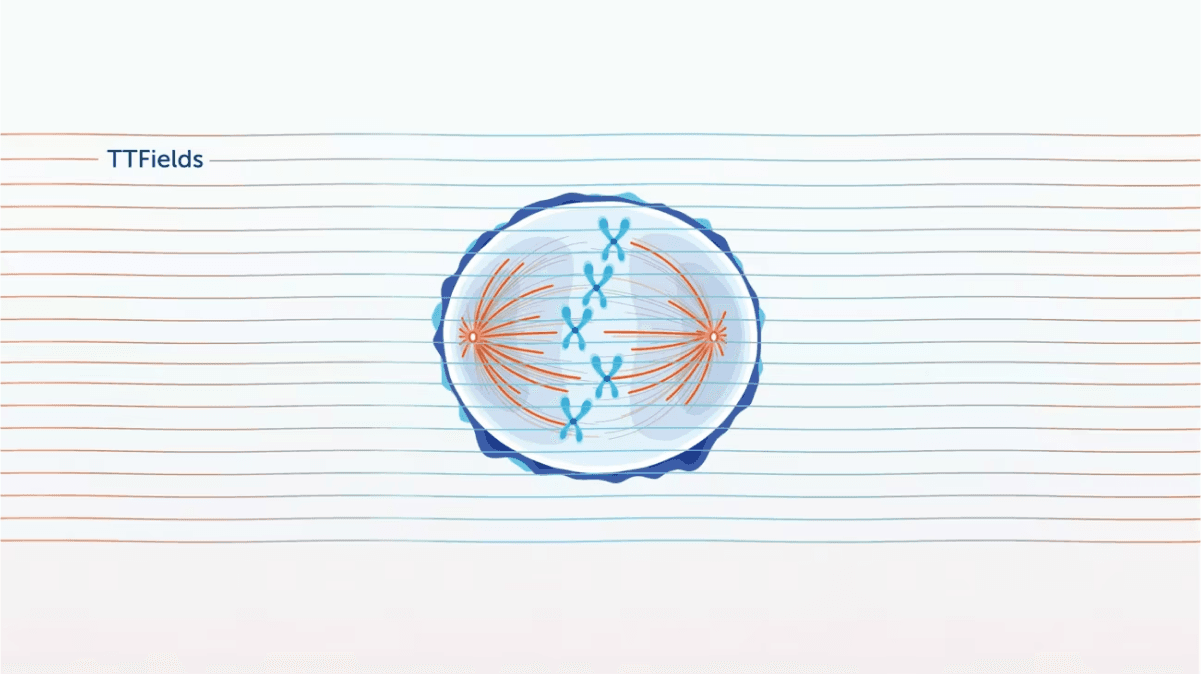
In preclinical models, TTFields have been shown to disrupt mitosis by exerting electrical forces on polar components involved in this process (e.g. spindle microtubules), thus disrupting their normal localization and function, ultimately leading to cell death.1-3
Explore each phase of mitosis to see the impact of TTFields

In metaphase:
TTFields impair microtubule assembly in cancer cells, leading to aberrant mitotic spindle formation.2,4

In anaphase:
TTFields disrupt the arrangement of septin at the anaphase cleavage furrow in cancer cells, inducing cytoplasmic membrane blebbing, mitotic failure, and asymmetric chromosome segregation.1,5

In telophase:
TTFields push polar organelles and macromolecules (a process called dielectrophoresis) to the bridge between the forming daughter cells, where - due to the hourglass shape - TTFields intensity is highest4,5


In preclinical models, TTFields have been shown to induce immunogenic cell death of cancer cells (e.g. release of HMGB1 from the nucleus, cell surface exposure of calreticulin, and autophagy-mediated ATP release), thus triggering an anti-cancer immune response
In preclinical models, TTFields have been shown to alter the organization and dynamics of the cytoskeleton, disrupting cancer cell mobility and migration, which are essential for metastasis.1
A model illustrating the mechanism by which TTFields modulates cancer cell mobility

- Microtubules are required to specify the direction of cell movement. GEF-H1 catalytic activity is downregulated through microtubule binding.1
- TTFields exert directional forces on polar tubulins, leading to their alignment in the direction of the field. This in turn, leads to the reorganization of the microtubule network, and release of GEF-H1.
- Free GEF-H1 activates the RhoA/ROCK signaling pathway, inducing changes in actin dynamics.
- Changes in actin dynamics induce formation of focal adhesions, which disrupt polarity and migration directionality.
In preclinical models, TTFields have been shown to downregulate expression of proteins from the FA-BRCA pathway, an important DNA damage response pathway
Watch below to see how TTFields disrupt DNA damage repair

References: 1. Karanam NK, Story MD. An overview of potential novel mechanisms of action underlying tumor treating fields-induced cancer cell death and their clinical implications. Int J Radiat Biol. 2021;97(8):1044-1054. doi:10.1080/09553002.2020.1837984 2. Voloshin T, Schneiderman RS, Volodin A, et al. Tumor treating fields (TTFields) hinder cancer cell mobility through regulation of microtubule and actin dynamics. Cancers (Basel). 2020;12(10):1-18. doi:10.3390/cancers12103016 3. Mun EJ, Babiker HM, Weinberg U, Kirson ED, Von Hoff DD. Tumor-treating fields: a fourth modality in cancer treatment. Clin Cancer Res. 2018;24(2):266-275. doi:10.1158/1078-0432.CCR-17-1117 4. Cooper GM. The development and causes of cancer. In: The Cell: A Molecular Approach. 2nd ed. Sinauer Associates; 2000:chap 15. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK9963/ 5. Baba AI, Câtoi C. Tumor cell morphology. In: Comparative Oncology. The Publishing House of the Romanian Academy; 2007:chap 3. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK9553/ 6. Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:1-16. doi:10.1038/srep18046 7. Kirson ED, Dbalý V, Tovaryš F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104(24):10152-10157. doi:10.1073/pnas.0702916104 8. Ablation for liver cancer. American Cancer Society. Updated April 1, 2019. Accessed June 21, 2022. https://www.cancer.org/cancer/liver-cancer/treating/tumor-ablation.html 9. Krauss JK, Lipsman N, Aziz T, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17(2):75-87. doi:10.1038/s41582-020-00426-z 10. Mulpuru SK, Madhavan M, McLeod CJ, Cha Y-M, Friedman PA. Cardiac pacemakers: function, troubleshooting, and management. Am Coll Cardiol. 2017;69(2):189-210. doi:10.1016/j.jacc.2016.10.061 11. Wenger C, Giladi M, Bomzon Z, Salvador R, Basser PJ, Miranda PC. Modeling Tumor Treating Fields (TTFields) application in single cells during metaphase and telophase. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:6892-6895. doi:10.1109/EMBC.2015.7319977 12. Trainito CI, Sweeney DC, Čemažar J, et al. Characterization of sequentially-staged cancer cells using electrorotation. PLoS One. 2019;14(9):1-18. doi:10.1371/journal.pone.0222289 13. Haemmerich D, Schutt DJ, Wright AW, Webster JG, Mahvi DM. Electrical conductivity measurement of excised human metastatic liver tumours before and after thermal ablation. Physiol Meas. 2009;30(5):459-466. doi:10.1088/0967-3334/30/5/003